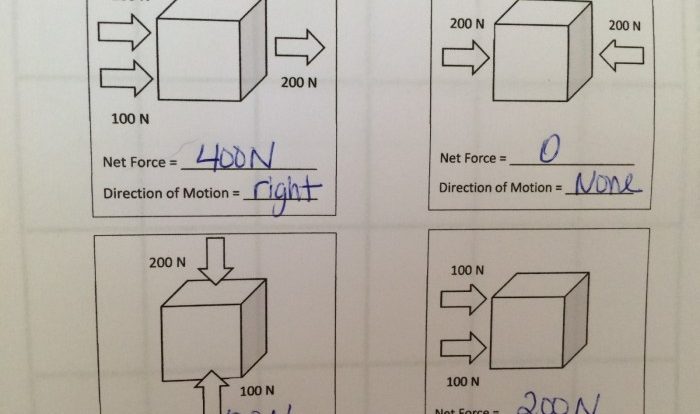
Conceptual physics practice page chapter 14 gases gas pressure answers – Delving into the realm of conceptual physics, this practice page on gases, gas pressure, and their applications offers an immersive exploration into the fundamental principles that govern the behavior of gases. Through engaging discussions, real-world examples, and interactive simulations, we embark on a journey to unravel the mysteries of gas pressure, gas laws, and their widespread applications across various scientific and engineering fields.
This comprehensive resource provides a thorough understanding of the concepts covered in Chapter 14, equipping students with a solid foundation in gas physics. By exploring the intricate relationship between gas pressure, volume, temperature, and particle count, we gain insights into the behavior of gases under varying conditions.
1. Introduction to Conceptual Physics Practice Page Chapter 14
Conceptual Physics Practice Page Chapter 14 provides students with an interactive and engaging way to learn about the fundamental concepts of gases, gas pressure, and gas laws.
The chapter covers topics such as the definition of gas pressure, factors affecting gas pressure, Boyle’s Law, Charles’s Law, the Combined Gas Law, and applications of gas principles in various fields.
2. Understanding Gas Pressure

Gas pressure is defined as the force exerted by a gas per unit area. It is a measure of the intensity of the force exerted by the gas molecules on the walls of their container.
Factors that affect gas pressure include temperature, volume, and the number of gas particles. Increasing the temperature or decreasing the volume of a gas will increase its pressure. Increasing the number of gas particles in a given volume will also increase its pressure.
3. Exploring Gas Laws
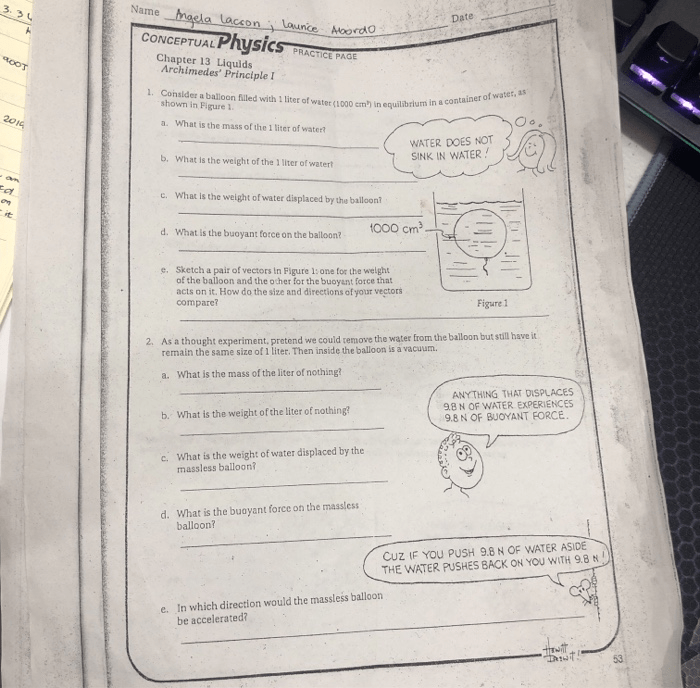
Gas laws describe the behavior of gases under different conditions.
- Boyle’s Law states that the pressure of a gas is inversely proportional to its volume at constant temperature.
- Charles’s Law states that the volume of a gas is directly proportional to its temperature at constant pressure.
- The Combined Gas Law combines Boyle’s Law and Charles’s Law to describe the behavior of gases under changing conditions of pressure, volume, and temperature.
4. Applications of Gas Principles
Gas pressure and gas laws have numerous applications in various fields:
- Meteorology: Atmospheric pressure and weather patterns
- Engineering: Designing pressure vessels and gas turbines
- Medicine: Respiratory system and anesthesia
- Environmental science: Pollution control and greenhouse gases
5. Practice Problems and Solutions: Conceptual Physics Practice Page Chapter 14 Gases Gas Pressure Answers
Practice problems are provided to reinforce students’ understanding of gas pressure and gas laws.
Detailed solutions and explanations are offered for each problem, encouraging students to apply their knowledge to solve real-world problems.
6. Interactive Simulations and Visualizations
Interactive simulations and visualizations are incorporated to illustrate the concepts of gas pressure and gas laws.
Students can explore the effects of changing variables and observe the corresponding changes in gas behavior.
FAQ Summary
What is the relationship between gas pressure and temperature?
According to Charles’s Law, the pressure of a gas is directly proportional to its absolute temperature, assuming constant volume.
How does Boyle’s Law describe the behavior of gases?
Boyle’s Law states that the pressure of a gas is inversely proportional to its volume, assuming constant temperature.
What are some real-world applications of gas pressure?
Gas pressure finds applications in various fields, including the design of pressure vessels, operation of gas turbines, weather forecasting, and medical procedures such as anesthesia.