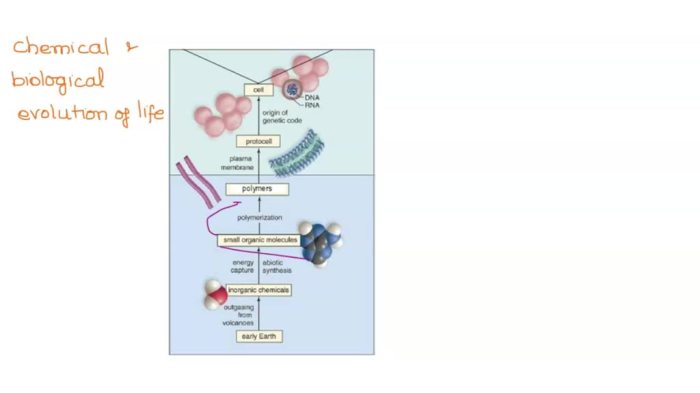
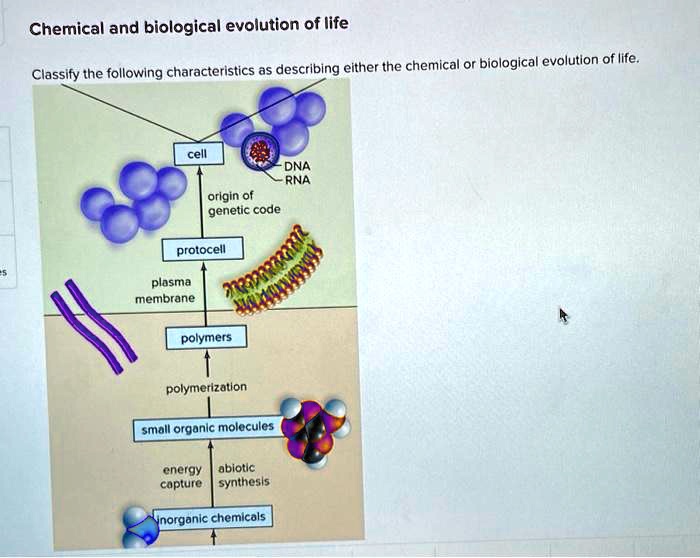
Classify the compounds as either polymers or not polymers – In the realm of chemistry, discerning between polymers and non-polymers is a crucial task, enabling scientists and researchers to categorize compounds based on their distinct characteristics. This classification serves as a foundation for understanding material properties, predicting performance, and guiding applications in diverse fields.
Polymers, characterized by their high molecular weight and repeating structural units, exhibit unique properties that set them apart from non-polymeric compounds. The classification criteria encompass molecular weight thresholds, repeating unit composition, and factors influencing polymerization degree and chain length.
2. Criteria for Classification
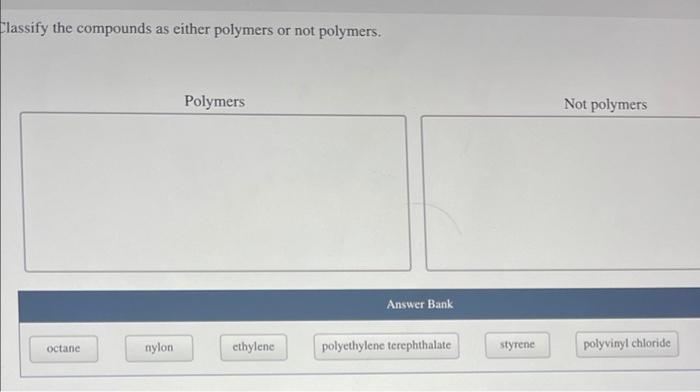
To distinguish polymers from non-polymers, several criteria are considered:
Molecular Weight Threshold
A key criterion is molecular weight. Generally, compounds with a molecular weight above 10,000 g/mol are considered polymers.
Repeating Units, Classify the compounds as either polymers or not polymers
Polymers consist of repeating units, which are small molecules that form the backbone of the polymer chain. These repeating units can be identical or different, leading to different types of polymers.
Other Factors
Additional factors that influence classification include the degree of polymerization (number of repeating units) and chain length. Higher degrees of polymerization and longer chain lengths typically indicate polymeric behavior.
3. Examples of Polymers
Below is a table showcasing common polymers and their properties:
| Polymer | Chemical Structure | Applications |
|---|---|---|
| Polyethylene (PE) | -(CH2-CH2)-n | Plastic bags, bottles, packaging |
| Polyvinyl Chloride (PVC) | -[-CH2-CHCl-]-n | Pipes, flooring, siding |
| Polystyrene (PS) | -[-CH(CH3)-CH2-]-n | Disposable cups, food containers |
| Polytetrafluoroethylene (PTFE) | -[-CF2-CF2-]-n | Non-stick cookware, seals |
4. Examples of Non-Polymers
The following table lists non-polymers with their structures and properties:
| Compound | Chemical Structure | Properties |
|---|---|---|
| Sodium chloride (NaCl) | NaCl | Ionic solid, soluble in water |
| Glucose | C6H12O6 | Sugar, soluble in water |
| Water (H2O) | H2O | Liquid, essential for life |
| Carbon dioxide (CO2) | CO2 | Gas, essential for photosynthesis |
FAQ Compilation: Classify The Compounds As Either Polymers Or Not Polymers
What is the significance of molecular weight in polymer classification?
Molecular weight serves as a key criterion for distinguishing polymers from non-polymers. Polymers typically possess high molecular weights, often exceeding 10,000 g/mol, while non-polymers generally have lower molecular weights.
How do repeating units contribute to polymer structure and properties?
Repeating units are the fundamental building blocks of polymers. The sequence, arrangement, and composition of these units determine the polymer’s structure, properties, and behavior. They influence factors such as crystallinity, flexibility, and thermal stability.
What are some examples of common polymers and their applications?
Polyethylene (PE) is a widely used polymer known for its strength, flexibility, and resistance to chemicals. It finds applications in packaging, construction, and automotive industries. Polyvinyl chloride (PVC) is another versatile polymer employed in pipes, flooring, and window frames due to its durability and resistance to fire and chemicals.